Silver fluoride and Tooth Dental
What is Silver Fluoride?
Silver fluoride (AgF) is a chemical compound that combines silver and fluoride ions. It appears as a white crystalline solid and dissolves easily in water. This dental compound is widely used for cavity prevention and tooth enamel protection.
How Silver Fluoride Works for Cavity Prevention
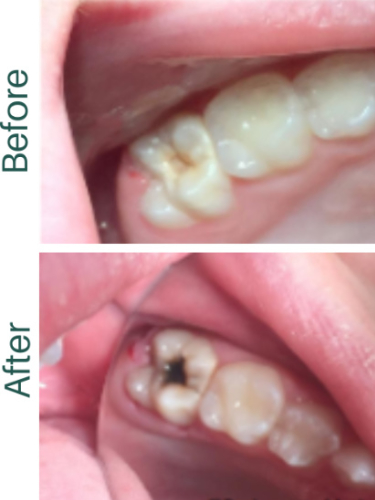
Silver fluoride is primarily used in dentistry as a topical cavity prevention treatment. When applied as a solution or gel, it interacts with tooth enamel, forming a protective silver phosphate layer. This layer strengthens the teeth and makes them highly resistant to acid attacks from harmful bacteria, helping to prevent cavities and tooth decay.
Key Benefits of Silver Fluoride in Dentistry
Silver fluoride offers multiple oral health benefits, including:
-
Cavity Prevention – Creates a protective barrier against enamel erosion.
-
Tooth Enamel Strengthening – Enhances enamel structure for better durability.
-
Reduction of Tooth Sensitivity – Helps protect nerve endings and reduce discomfort.
-
Remineralisation Support – Restores essential minerals like calcium and phosphate to improve tooth health.
-
Antimicrobial Properties – Prevents bacterial growth, lowering the risk of gum disease and dental infections.
Safety and Precautions
While silver fluoride is an effective dental treatment, it can be toxic if ingested in large amounts or inhaled as dust. To ensure safe usage, it should only be applied by a dental professional. Proper handling and storage are essential to avoid potential risks.
Why Silver Fluoride is an Effective Dental Treatment
Dentists recommend silver fluoride for cavity prevention, tooth remineralization, and long-term enamel protection. Its antimicrobial properties make it a valuable addition to preventive dental care, ensuring stronger, healthier teeth.
Learn More About Silver Fluoride and Dental Health
To explore more about silver fluoride treatments or to schedule a dental consultation, contact us on 3366 1737.
Pros





Cons




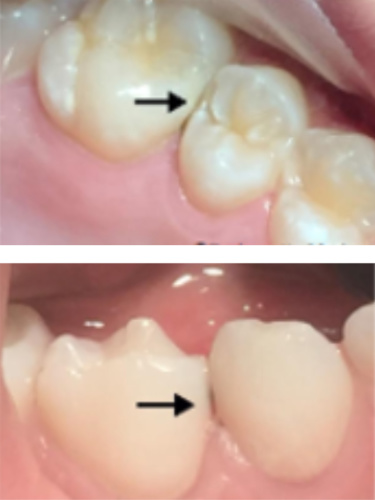
Silver Fluoride
treated molar
Silver Fluoride treated interproximal lesion